Currently many industrial and threshold countries work on a reorientation of their energy systems. Numerous states are strenghtening their investments in renewable energy sources, i.e. wind turbines or solar plants for the production of electrical energy. Particularly the production of electrical energy using wind or solar energy is fluctuating with day time and weather conditions. While there is an overproduction of electrical energy on windy or sunny days, the production of electricity is respectively low at night or under unfavourable weather conditions. Consequently there is an imbalance between the time of the energy production and its consumption.
One possibility to deal with this discrepancy is the power-to-gas process. The production of excess electrical energy enables the economic production of hydrogen by electrolysis. This hydrogen can either be used directly or be converted into liquid fuels or chemical raw materials. The two last-mentioned options enable the storage and the transport of hydrogen if there is no infrastructure for the useage of hydrogen close to the electrolysis plant available.
One option to store the produced hydrogen is the use of the existing natural gas grid. However, due to different combustion properties, the addition of hydrogen to the gas grid is currently legally limited. To utilize the existing natural gas grid nevertheless it is favourable to convert hydrogen to methane using a carbon source. This so called Synthetic Natural Gas (SNG) shows similar combustion properties to natural gas and can therefore be fed into the existing natural gas grid without legal restrictions.
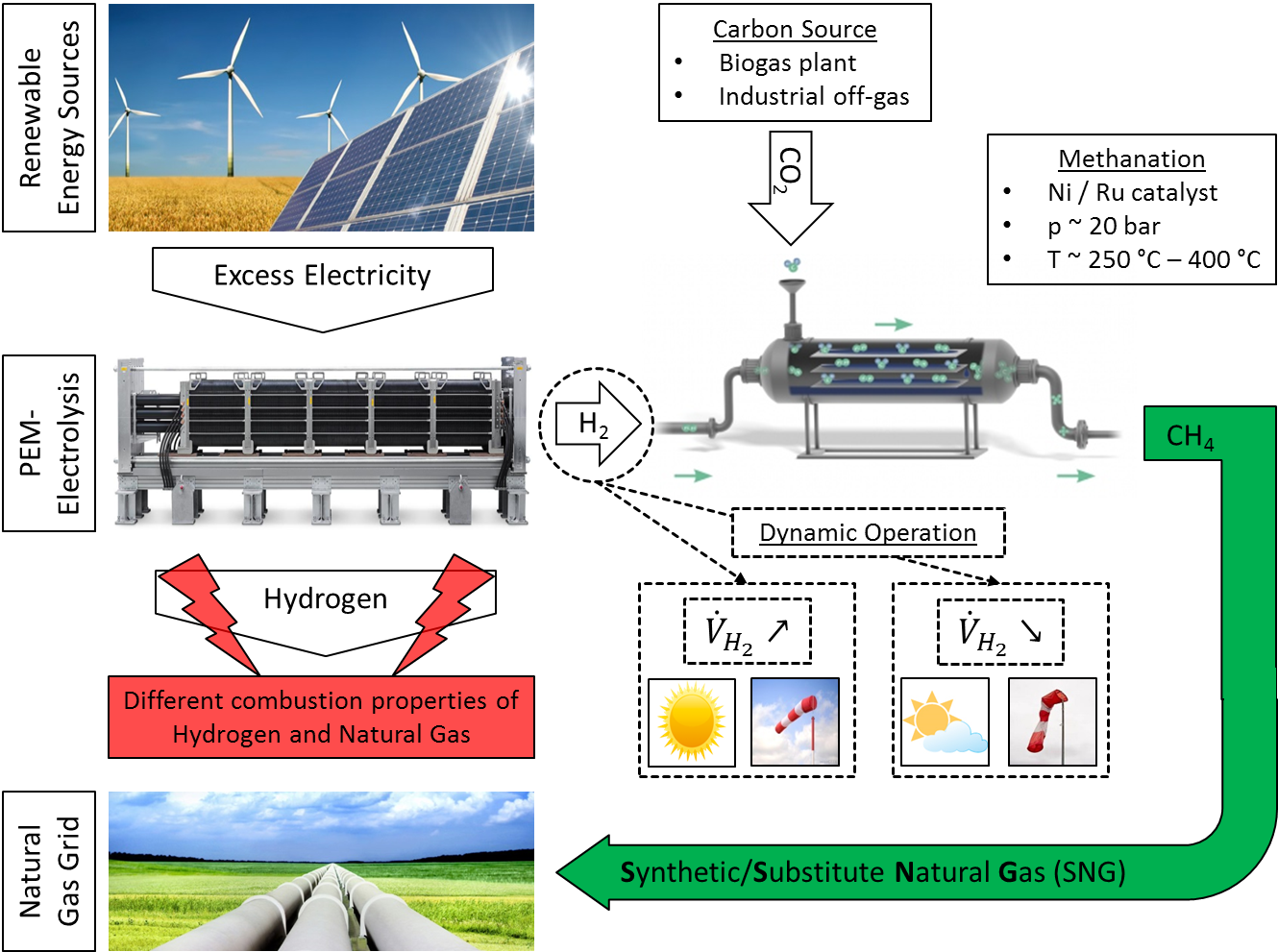
The fluctuating electrical energy production from renewable energy sources leads to an unsteady hydrogen output of the electrolysis. This unsteady hydrogen production puts high requirements on the dynamic operation of the methanation reactor. Frequent load changes could compromise the gas quality of the produced SNG or the stability of the catalyst. The aim of this research project is the development of a reactor concept able to handle the fluctuating hydrogen production to ensure a continuous feed of the SNG into the existing natural gas grid.
