OxFA-process- Oxidative conversion of biomass to formic acid
A promising alternative value chain for biomass could be the selective catalytic oxidation of biogenic feedstock in the OxFA process (Oxidation of Biomass to Formic Acid). This concept deals with a thermo-chemical transformation of biomass in a “cold and wet” combustion to yield formic acid (FA). This compound can act as a carbon dioxide based hydrogen equivalent and thus plays an important role as chemical hydrogen carrier molecule. Formic acid is comparable to conventional liquid fuels due to its liquid state and stability at ambient conditions with regards to handling, transport and storage. Furthermore, FA offers several opportunities for utilization in, for example, the livestock feed, pharmaceutical, textile or leather industries. Additionally, their salts can be used as de-icing agents at airports or as drilling fluid additives.
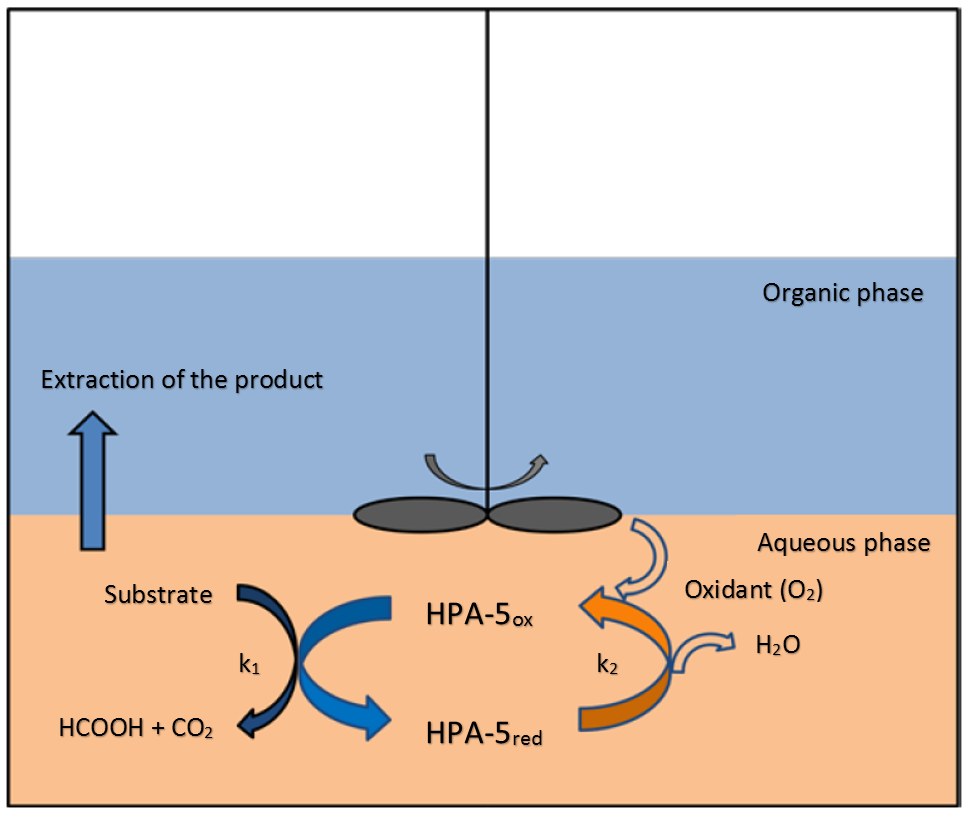
To make this chemical transformation possible, water-soluble, selective oxidation catalysts from the substance class of polyoxometalates are used. Their ability to oxidize a wide variety of sugar-containing biomass in aqueous suspension by the help of molecular oxygen and organic sulfonic acids as additives selectively to formic acid makes this unique process possible. Furthermore, molecular optimization of the catalyst structure can lead to an improved process management under extremely mild conditions in an exothermic reaction. In the presence of a second, organic phase the formic acid can be extracted from the aqueous reaction phase by an in-situ extractive distillation.
2011 the research results led to the patenting of the OxFA process. In March 2015, Dr. Wolf Ibach set up the OxFA® GmbH to continue the development of the process. The company was founded as a joint venture of IBACH GmbH and EnviTec Biogas AG.
In the research group “biomass and sustainable production of platform chemicals” we are developing the OxFA process to the industrial use. Therefore, an upscaling was performed and a continuous operating plant was built. By performing a detailed sensitivity analysis, the process parameters of this miniplant have already been optimized. Examination of various parameters (such as temperature, pressure, stirrer speed, concentration, volume flows and volume flow ratios) allowed us to determine the most important influencing factors for the process. A recycling of the extractant could be implemented as well. Even the conversion of real biomass is possible. In addition to model substrates such as sucrose, the oxidation of molasses to formic acid has recently been successfully completed.
