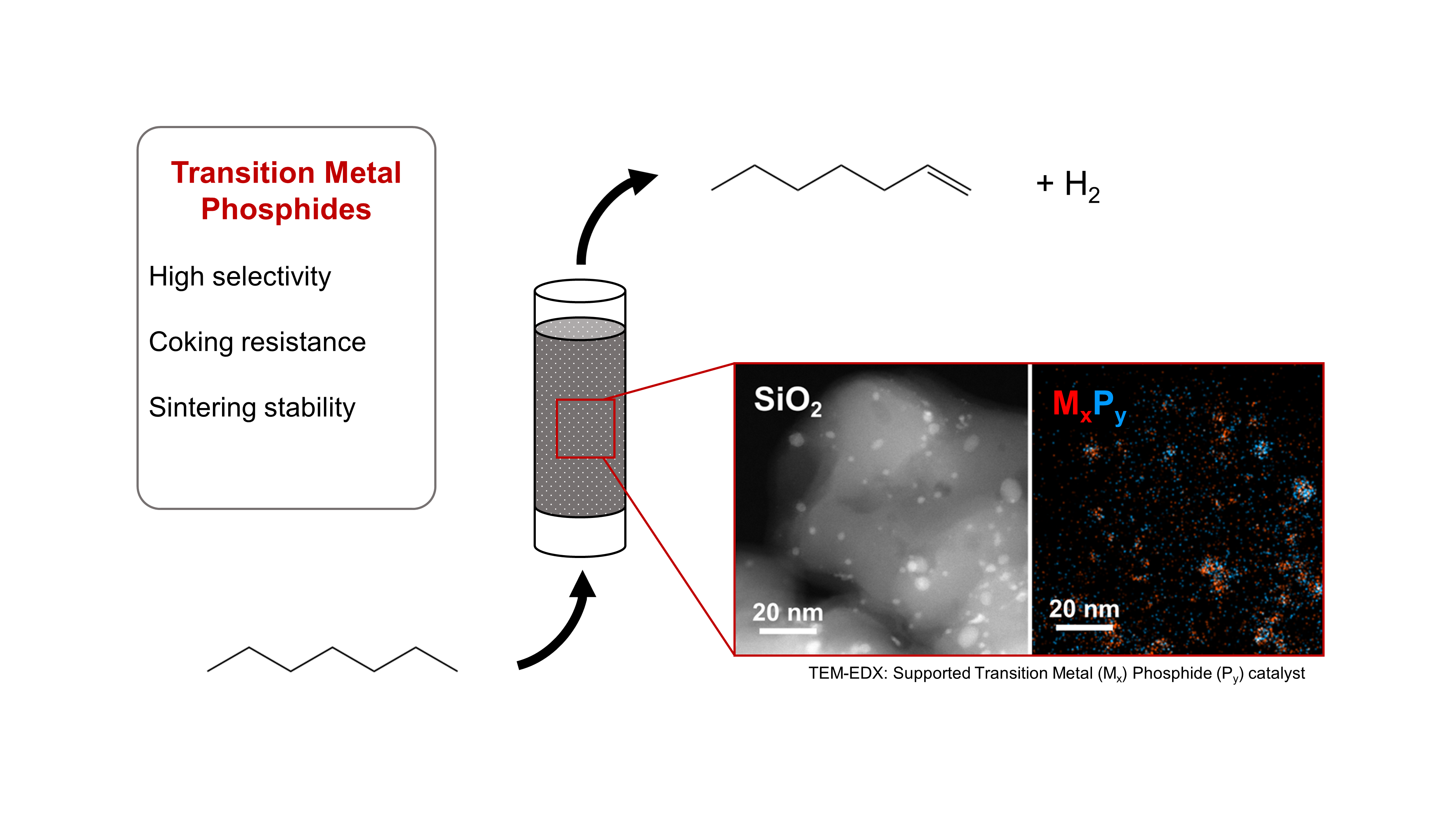
With the increasing shift towards renewable energy sources, medium-chain alkanes (C5-C10) derived from Fischer-Tropsch and biofuel synthesis processes are gaining importance as base chemicals for the chemical industry. The dehydrogenation of these alkanes to olefins opens up numerous reaction pathways for subsequent functionalization. As the chain length of the substrate alkanes increases, side reactions such as hydrogenolysis, isomerization, and dehydrocyclization become more prevalent, which increases the demands for highly selective and coke-resistant catalysts.
Traditional catalyst materials for alkane dehydrogenation often rely on expensive noble metals like platinum or palladium. As a cost-effective alternative, transition metals such as nickel, molybdenum, iron, and cobalt also exhibit catalytic properties but are often less effective in terms of activity, selectivity, and stability. By modifying the transition metal species with sterically and electronically effective phosphorus, the bonding behavior (coordination and bond strength) to reactants and the catalytic surface properties can be influenced. The crystalline transition metal phosphides (TMPs) formed through phosphorus modification have already shown high selectivity and stability against coking and sintering in the dehydrogenation of short-chain alkanes (C2-C4) and n-heptane (C7). [1]
As part of a project funded by the German Research Foundation (DFG, from ger. Deutsche Forschungsgemeinschaft, project number: 537270564), TMPs are being specifically developed for the dehydrogenation of medium-chain alkanes. A novel low-temperature synthesis method for TMPs is employed, using phosphonium-based ionic liquids as both a substrate and a structure template.
The synthesized materials undergo extensive analyses to determine the elemental composition, crystal structure, and bonding information of the catalysts. Additionally, the TMP materials are studied during in situ sintering studies to investigate the positive effect of phosphorus modification on the sintering stability of the materials depending on the metal-phosphorus ratio. Complementarily, the resistance of the synthesized catalysts to coking and possible reductive/oxidative regeneration of the material is examined using catalysts tested in dehydrogenation.
Furthermore, applied reaction studies of the synthesized TMP catalysts are conducted using a fully automated pilot plant for continuous gas-phase dehydrogenation of medium-chain alkanes. n‑Heptane and Methylcyclohexane are used as model substrates to demonstrate the catalytic properties of TMPs for the dehydrogenation of linear and cyclic alkanes. The focus of these studies is to investigate the influence of phosphorus modification on the activity, selectivity, and stability of the transition metal catalysts. As part of the reaction engineering testing, the synthesized TMP catalysts are compared with conventional catalysts for alkane dehydrogenation to enable a comprehensive evaluation of the novel catalyst materials.
Ultimately, synthesis-structure-activity relationships are to be derived from the insights gained through comprehensive characterization and reaction engineering studies, contributing to a better understanding of TMP materials.
[1] Stöber, R.; Mai, F.; Sebastian, O.; Körner, A.; Hutzler, A.; Schühle, P. A Highly Stable Bimetallic Transition Metal Phosphide Catalyst for Selective Dehydrogenation of N‐ Heptane. ChemCatChem 2022, 14 (18). https://doi.org/10.1002/cctc.202200371.
