Synthesis of dimethyl carbonate from renewable raw materials
Climate change is one of the biggest challenges of this century, largely due to CO2-emissions. To reduce emissions from burning fossil fuels, renewable processes for synthesizing additive fuels need to be developed and tested. An attractive fuel additive is dimethyl carbonate (DMC) as it burns with low formation of soot. DMC is a low hazard liquid with a high volumetric energy density. Within the framework of this project, the synthesis of DMC from biomass-derived raw materials is being investigated. Two synthesis routes, which are illustrated in Figure 1, will be studied.
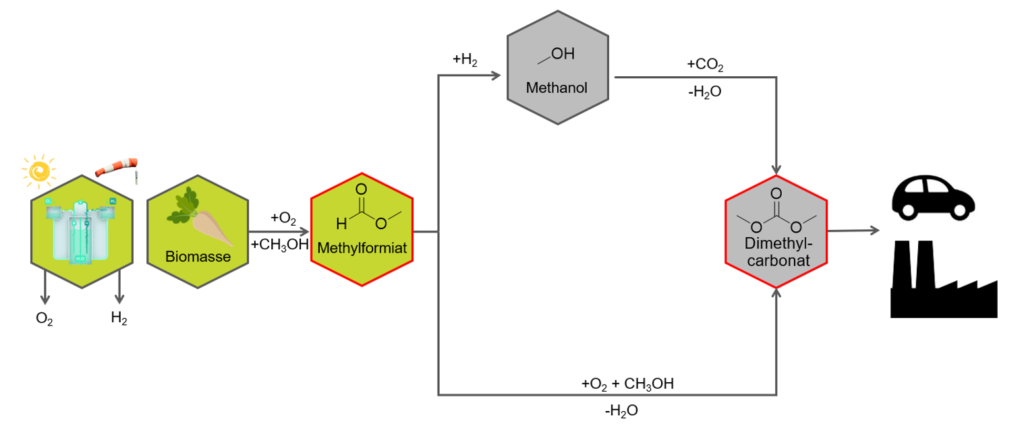
Figure 1: Synthetic routes from biomass to DMC
The starting materials for DMC synthesis are methyl formate and methanol, which were previously obtained in the OxFA-process by the oxidative conversion of sugar-containing biomass [1]. DMC is synthesized in an oxidative step using oxygen or air. In addition to developing highly active and selective catalysts, it is important to etablish concepts for heat dissipation, considering the high safety requirements of the exothermic reaction.
In an alternative process, the reaction of methanol with CO2 to form DMC and water is carried out. Here, biomass-derived CO2 is used and converted into a valuable product. However, since CO2 is thermodynamically stable under normal conditions, equilibrium conversions of < 5% are achieved in this reaction [2]. The integrated removal of the by-product water using suitable dehydrants allows the thermodynamic equilibrium to be shifted further in the direction of the products.
The processes for the synthesis of renewable fuel additives from biomass-derived raw materials are compared within the framework of an economic analysis. The project is carried out in cooperation with the Helmholtz Institute Erlangen-Nuremberg (HIERN) and is funded by the Bavarian State Ministry for Economic Affairs, Energy and Technology (StMWi).
[1] Maerten, Stephanie; Kumpidet, Chiraphat; Voß, Dorothea; Bukowski, Anna; Wasserscheid, Peter; Albert, Jakob (2020): Glucose oxidation to formic acid and methyl formate in perfect selectivity. In: Green Chem. 22 (13), S. 4311–4320. DOI: 10.1039/D0GC01169J.
[2] Zhang, Meng; Xu, Yonghang; Williams, Brandon L.; Xiao, Min; Wang, Shuanjin; Han, Dongmei et al. (2021): Catalytic materials for direct synthesis of dimethyl carbonate (DMC) from CO2. In: Journal of Cleaner Production 279, S. 123344. DOI: 10.1016/j.jclepro.2020.123344.
